- Profile
- Comments 0
- prev
- next
- Website
- Leave a comment
- prev
- next
In a nutshell
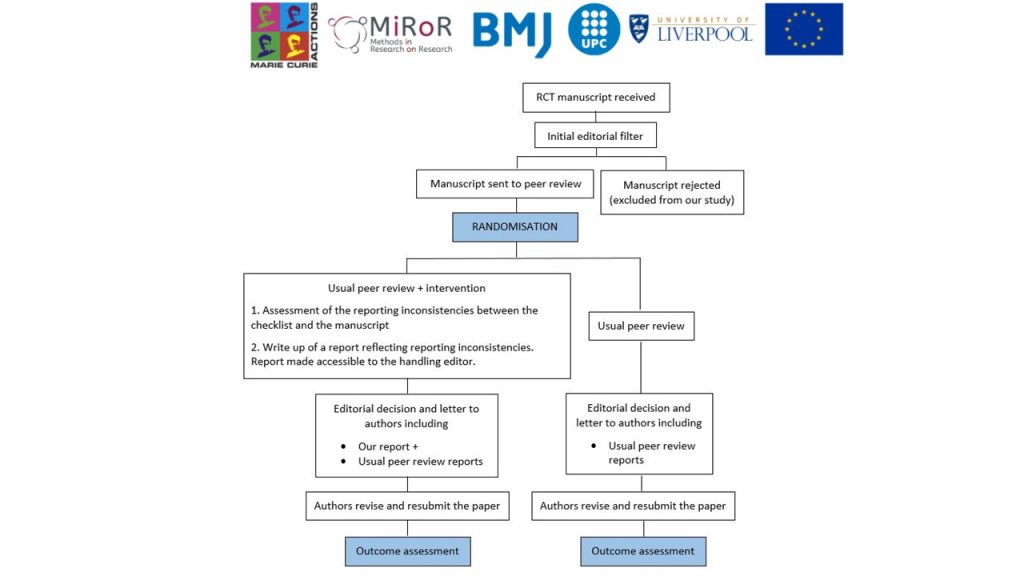
Randomised controlled trial in collaboration with BMJ Open, a general medical journal, to evaluate in a real editorial context whether assessing during peer review the consistency between the submitted CONSORT checklist and the information reported in the manuscripts of randomised trials, as well as providing feedback to authors on the inconsistencies found, improves the completeness of reporting of published trials.
Goals and intentions
Randomised trials are considered the gold standard in medical research. The CONSORT Statement aims to improve the quality of reporting of randomised trials. Without transparent reporting, readers cannot judge the reliability of trial findings, therefore these findings cannot inform clinical practice. Different stakeholders, including biomedical journals, have taken different actions to try to improve author adherence to CONSORT. The most popular one is to instruct authors to submit a completed CONSORT checklist with page numbers indicating where the CONSORT items are addressed when they submit their manuscript. However, this measure alone has proven not to be effective. In this study, we intend to assess whether evaluating the checklists submitted by authors and providing feedback to them as part of the peer review process improves the completeness of reporting of published trials.
Review features
-
Manuscript hostingYes
-
Notes
Randomised controlled trials in the intervention group will receive an additional peer review (conducted by the principal investigator) focused entirely on the consistency of reporting between the submitted manuscript and the completed CONSORT checklist. Where inconsistencies are found, authors of these manuscripts will be asked for changes in relation to the items. Trials in the control group will undergo the usual peer review process.
-
Eligible reviewers/editorsEligibility criteria: Manuscripts are eligible for our study if (i) they have been submitted to BMJ Open, (ii) they are original research submissions reporting the results of a randomised trial and (iii) they have passed the first editorial filter and have been subsequently sent out for peer review.
Transparency
Results
-
Results summary
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 676207.
Results will be available n September 2019.
-
Preregistration URL

Add a comment